What You Will Learn
- Photons, tiny packets of light
- How photon energy relates to color
- Why some light can cause chemical and biological changes
Simple Explanation
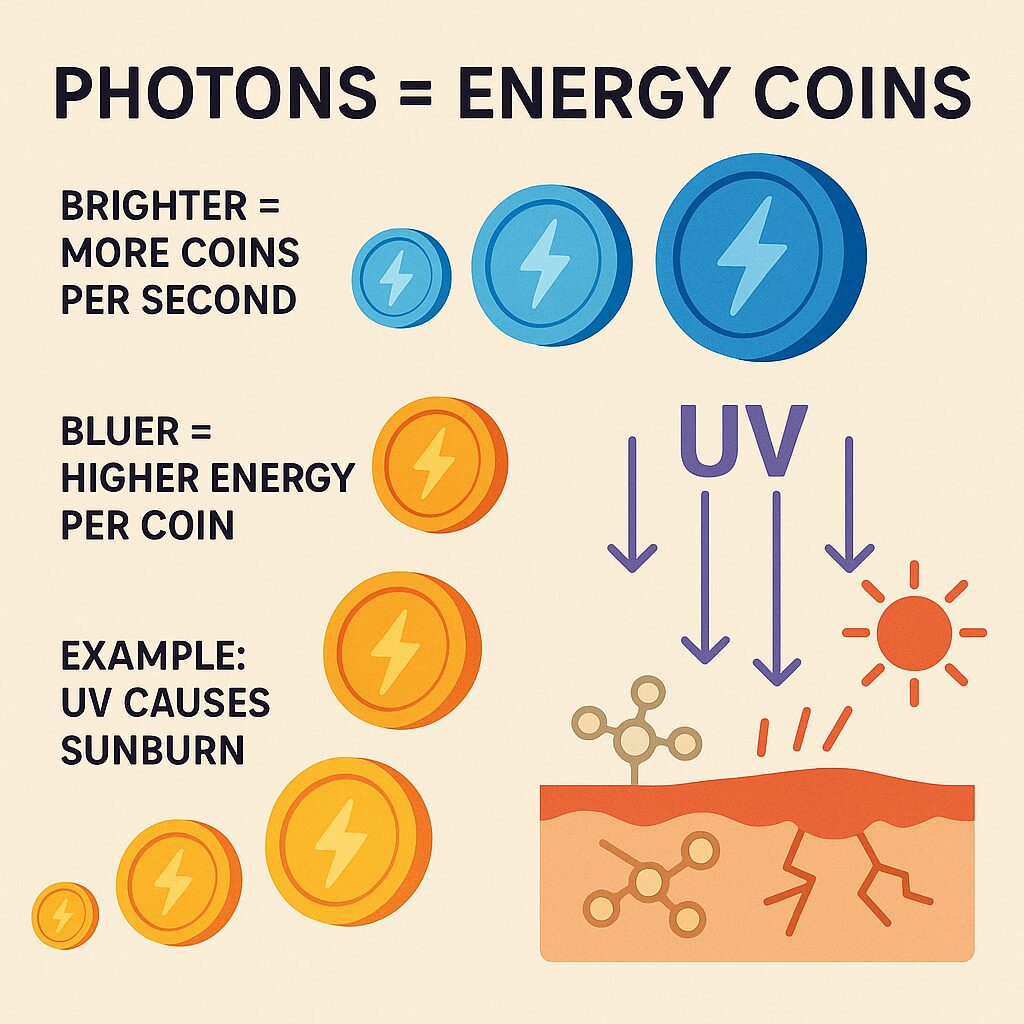 Light also behaves like particles called photons, think of them as tiny energy coins. Brighter light means more coins arriving each second, bluer light means each coin carries more energy than a red one.
Light also behaves like particles called photons, think of them as tiny energy coins. Brighter light means more coins arriving each second, bluer light means each coin carries more energy than a red one.
Example: Ultraviolet, UV, light can cause sunburn because each UV photon carries enough energy to disturb or break certain molecules in skin.
Deeper Explanation
Wave–particle duality means both pictures matter, we use the wave view to predict bending and interference, and the photon view to explain how energy transfers in chunks. Devices that measure light, photodiodes, camera pixels, photon counters, register individual photon arrivals. At very low light, the arrivals are random clicks, this creates fundamental shot noise. That is why very dim photos look speckled even with a perfect sensor, sometimes only a few photons arrive.
Photons interact with matter by giving energy to electrons in atoms and molecules. If a photon’s energy is high enough, it can kick an electron to a new state, start a chemical reaction, or damage biological molecules. That is the key difference between gentle warmth from infrared, low energy per photon, and UV’s ability to cause photochemical changes like tanning, sunburn, or DNA damage. The same principle enables useful effects, photosynthesis in plants, UV curing of adhesives, and the photoelectric effect in solar cells.
Math in Context
Energy per photon tracks color.

A handy rule is:
A shorter wavelength, or higher frequency, means higher energy per photon. Violet at 400 nm is about 3.1 eV per photon, deep red at 700 nm is about 1.8 eV. You do not need to memorize the constant, just remember that sliding toward violet and UV raises the energy carried by each photon.
What those numbers mean physically:
Many chemical bonds and electronic gaps in materials sit around 2 to 5 eV. Photons above the needed energy can trigger changes, below that, they usually just warm things. That is why UV, about 3 to 10 eV, can drive reactions or damage DNA, while infrared, less than about 1.5 eV, mainly heats by vibrating atoms rather than breaking bonds.
Bandgaps explain LEDs and solar cells.
Solids have a threshold energy called a bandgap. If a photon’s energy is above the gap, it can create an electron and hole pair, this is how solar cells work. If an electron drops across the gap, a photon can be emitted, this is how LEDs glow. Typical gaps, silicon about 1.1 eV, sensitive to red and near IR, gallium nitride, GaN based materials, about 2 to 3 eV, blue. That is why blue LEDs need wide gap semiconductors, and why silicon solar cells harvest red and near IR efficiently but do not absorb far IR or microwaves.
Photon count vs. photon energy, brightness vs. color: Color (wavelength) sets energy per photon. Brightness sets how many photons/second. A dim violet can eject electrons where a bright red cannot. Shorter wavelength (violet) means higher photon energy E=hc/λ. Even dim violet can eject electrons when E > φ. Brightness is mostly how many photons/second hit the cathode. Flux ≈ Power / Energy per photon. When E > φ, electrons are emitted and pulled to the anode, making a current that grows with brightness.
Brightness is mostly how many photons per second hit an area, color sets how much energy each photon carries. A dim violet LED has higher energy photons than a bright red LED, the violet just sends fewer of them. Engineers often calculate photon flux, photons per second, by dividing power by energy per photon.
Photoelectric Effect
Color → Energy per photon
Brightness → Photon flux
Photocurrent
Dose matters for biology:
Biological effects depend on photon energy and how many arrive, the dose, measured as energy per area. UV A photons have less energy than UV B photons, but a large enough number can still cause damage over time. This is why sunscreen is rated to limit the total harmful energy your skin absorbs, and why window glass, which blocks most UV B, reduces sunburn risk even though visible light still gets through.
Everyday tips to remember:
- Moving from red to blue to UV raises energy per photon.
- Moving from air into glass does not change photon energy, the color, but it changes speed and wavelength inside the material, the photon energy is set by the source.
- More power can mean more photons, higher energy photons, or both, know which one matters for your application, imaging, safety, or energy harvesting.
Why This Matters
Photon thinking explains why cameras count light in discrete steps, why low light images are noisy, why solar cells and LEDs work at particular colors, and why UV requires careful safety while infrared mainly heats. In design and safety, you will often ask two questions, how many photons are arriving, and how energetic is each one?
